Qualitative determination of food pH is probably the oldest analytical technique in the world. Foods are tested by taste organs. Some of these substances are acidic and others are alkaline. With the use of modern electrodes, it is possible to measure the pH of substances that are measured by the sense of taste with more accurate figures

Qualitative determination of food pH is probably the oldest analytical technique in the world. Foods are tested by taste organs. Some of these substances are acidic and others are alkaline. With the use of modern electrodes, it is possible to measure the pH of substances that are measured by the sense of taste with more accurate figures; You can see all kinds of portable ph meters , tabletop, pen, etc. on the website of Ario Exir Mandegar Company.

ph of different foods
The acidity or alkalinity of a solution depends on the concentration of hydrogen ions (H with a single positive ion) present in it. In the Sorenson equation, pH is defined as the negative logarithm of the hydrogen ion concentration in the desired solution. In other words, in high concentration (acidic state), pH = 0; and 10 to the power of zero = 1mol/L and in low concentration (play mode) PH=14; And 10 is to the negative power of 14 = 1mol/L. In this way, different materials can be objectively compared. At pH 0, the environment is very acidic, at pH 14, it is very alkaline, and at pH 7, the desired environment is neutral. In recent years, pH measurement has gained special importance. Measuring and monitoring the pH value is essential in controlling and regulating chemical and biological processes.
One of the types of pH meters is the inolab pH 7110 model , which is manufactured by the German company WTW . One of the most important features of this device is a large screen with the ability to simultaneously display pH and temperature, the possibility of 3-point calibration, the possibility of calculating 3 pH parameters, the ability to adjust and correct the temperature automatically, etc. In a dedicated article, we have described the Inolab pH 7110 device guide for you, which you can read.

PH scale
To measure the pH value, a detection electrode (pH meter electrode) and a reference electrode are required. In many cases, combination electrodes that incorporate both detector and reference elements are often used.
When the glass electrodes of the pH meter come into contact with the aqueous solution to be measured, a gel layer is formed on the pH-sensitive glass membrane. This gel layer, which is formed inside the glass membrane, is in contact with the internal buffer solution.

According to the pH of the desired solution, hydrogen ions with a positive charge are released out of the gel layer or transferred into it. In the alkaline (base) solution, the once positive hydrogen ions diffuse out of the gel layer and lead to a negative charge in the outer part of the gel layer. Since the glass electrode has an internal buffer with a constant pH, the total membrane potential is equal to the difference between the internal and external membrane potential.
![]()
where in:
Electrode potential = Eel; zero potential = E0; Slope (mV in pH unit) = S; Internal buffer pH = PHi; pH of the measured solution = PHa.


Overview of glass membrane performance
The pH meter circuit consists of a measuring electrode (glass electrode) and a reference electrode, both of which are immersed in a solution. To obtain the pH value, the reference electrode must have a stable and definite potential, which is independent of the calculated solution.


Each reference electrode contains a reference element that is immersed in a certain electrolyte. The electrolyte must always be in contact with the measured solution. This contact mainly occurs through the porous ceramic connection point. Among a number of reference systems, the mercury/calomel and silver/silver chloride systems have found special practical importance even though they have undergone certain changes. However, today, due to environmental considerations, mercury electrodes are rarely used to measure pH. The potential of the reference electrode system is determined by the reference electrolyte (silver/silver chloride). It is very important to have a high ion concentration in the reference electrolyte; Because it leads to a decrease in electrical resistance. Ideally, no reaction should occur over a wide temperature range between the reference electrolyte and the measured solution.
The use of combined electrodes has become popular due to their easier control than separate electrodes. In composite electrodes, the glass electrode is concentrically surrounded by the reference electrolyte. Only when different parts of the electrode have different average lifetimes, it is recommended to use separate electrodes instead of a combined electrode in measuring the pH of different materials.
One of the recent innovations is the addition of a temperature sensor to the combined pH electrodes. By placing the temperature sensor in the body where pH and reference elements are embedded, the temperature can be seen simply by checking the sensor.

Composite electrode structure
Successful pH measurement is only possible by choosing the right system to meet the needs of the sample. It is also very important to choose the right device and prepare the right reagents.
When choosing a pH measurement system, the following should be considered:
For optimal pH measurement, the right electrode must be selected first. The following are considered for electrode selection: chemical composition, homogeneity, temperature, pH range, and chamber size (length and width limitations). In non-aqueous, low-conductivity, protein-rich, and viscous samples, where glass electrodes are generally exposed to various sources of error, choosing the right electrode is particularly important. The response speed and accuracy of the electrode depends on various factors. The time required to measure a sample at high pH and temperature or low conductivity may be longer than the time required to measure aqueous solutions at room temperature and neutral pH.
The accuracy of the pH displayed by the electrode depends on the continuous stability of the electrode as well as laboratory factors such as temperature, cleanliness and freshness of buffer solutions and sample condition. The pH meter electrode is identified by the zero point and slope. Two-point calibration is used to increase measurement accuracy.
The pH values obtained from the electrode are defined by the Nernst equation.

In the above equation, E0 is a constant value, R is a gas constant, F is a Faraday constant, T is a Kelvin temperature, and n is an ion change.
When measuring hydrogen ions (n=±1) at a temperature of 25˚C (298K), the slope coefficient is equal to 59.16 mV. This value is considered the ideal slope coefficient. As a result, by changing one pH unit in the measurement system, the ideal slope coefficient changes by one millivolt. By measuring the slope coefficient, the functioning of the electrode system is determined.
If the electrodes are not cleaned and disinfected in the long term after use, the accuracy of the system will gradually decrease and disappear. System malfunction can be controlled by continuously decreasing the slope.
When the slope value reaches less than 50 mV per decade (85% slope efficiency) or the drift at the zero point reaches ±30 mV, this large change may restore the performance of the electrodes to the desired level, but in order to achieve greater accuracy In pH measurement, it is necessary to change the electrode.
However, it should be remembered that factors such as blocking of the reference junction, loss of electrolyte, contamination of the glass bulb, and the use of inappropriate calibration buffers lead to a decrease in the scale of the slope. If there is a disturbance in the functioning of the pH system and the correctness of the obtained pH cannot be ensured, the above items should be carefully examined.
The automatic temperature corrector (ATC) is generally used in laboratories except when both the calibration and the pH value are measured at a constant temperature. If the difference between the temperature of the sample and the calibration temperature is more than 10˚C, the absence of a suitable temperature will lead to an error of 0.15 units or more in the obtained pH values (between pH 3 and 11).
The pH range (0-14) is determined by the ionic product of water. Water partially dissociates into H+ and -OH ions.
![]()
The product ion I is strongly dependent on temperature. Temperature affects pH values through the following four factors:
Every solution has a certain temperature and pH (temperature coefficient). Due to the dependence of the separation of molecules on temperature, which causes a change in concentration. As a result, temperature change leads to pH change. This type of change is real and is not considered a measurement error; Therefore, it cannot be corrected using ATC either.
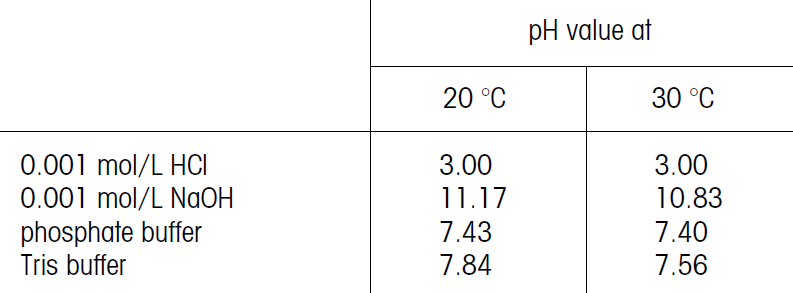
In comparing the pH values obtained at different temperatures, the above should be considered. Practically, the samples should be measured at the same temperature.
In the Nernst slope coefficient equation, the temperature term is also observed.
![]()
When measuring pH, the effects of temperature can be controlled and neutralized in various ways. Most pH meters have manual or automatic temperature correctors (ATC). A temperature sensor (ATC) is installed in the body of the pH electrode to measure the temperature. According to the Nernst equation, ATCs measure the temperature of the solution and pH meters display the pH value electronically.
If the calibration lines (isothermal) intersect at the zero point of the electrode (0 mV=pH 7) at different temperatures, the desired electrode will have an ideal temperature.

Intersection table of calibration lines and isothermal points
Because the total potential of the pH electrode consists of the sum of individual potentials and each potential has different temperature dependencies. Therefore, the isothermal intersection rarely coincides with the zero point of the electrode (ideal state: 0 mV at pH=7.25˚C).
In recent years, electrode development has focused on reducing the distance between the isothermal junction and the zero point; Because as pH approaches 7, the temperature error becomes smaller. As the temperature difference between calibration and sample solutions increases, the measurement error increases. As a general rule, measurement errors occur mainly in 0.1 pH units. The most accurate pH value is obtained when the calibration temperature and the measured solutions are the same.
When the temperature of the environment changes rapidly, a conventional unit electrode changes the temperature of the electrode and makes it the same temperature as the environment. In order to increase the reaction speed of the composite electrode to environmental temperature changes, the temperature of the internal pioneer element and the external reference element must be permanently the same. The temperature dependence of the pioneer elements should alternately be equal to zero.
Suitable electrodes for pH measurement are distinguished in the first step by the mutual increase or decrease of the temperature of the output elements. These electrodes have the same temperature coefficient and isothermal intersection at pH 7 and 0 mV; Therefore, responding to temperature changes is done in the shortest possible time.

Temperature-time performance of composite electrodes
