Nitric acid is a chemical compound with the formula HNO 3 and a molecular mass of 63.01g/mol. Nitric acid has a very high acidity ability and therefore its dissolution ability is widely used in the industry. Other names are nitric acid, salt essence and acid. Pure nitric acid is a colorless, light-sensitive liquid that decomposes over time and turns into nitrogen oxides and finally changes color f
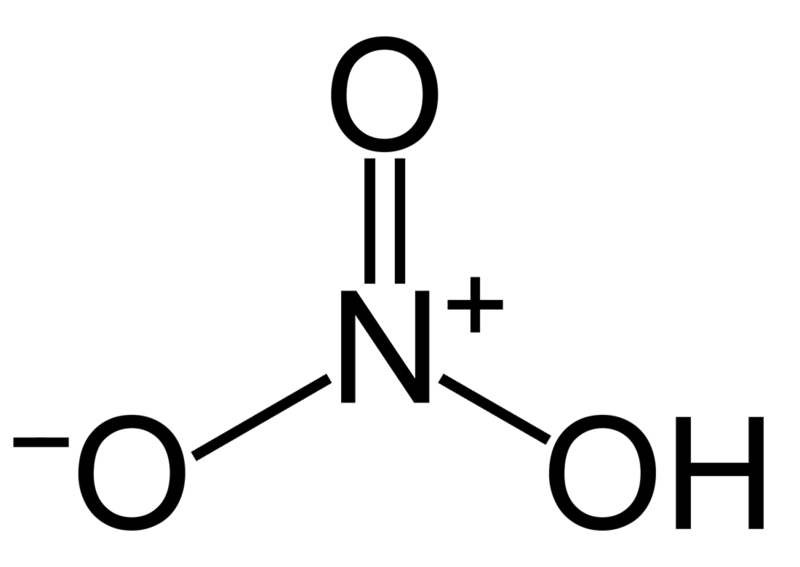
Nitric acid is a chemical compound with the formula HNO 3 and a molecular mass of 63.01g/mol. Nitric acid has a very high acidity ability and therefore its dissolution ability is widely used in the industry. Other names are nitric acid, salt essence and acid. Pure nitric acid is a colorless, light-sensitive liquid that decomposes over time and turns into nitrogen oxides and finally changes color from yellow to red.
Another interesting feature of nitric acid is that its small amount is edible. Nitric acid is soluble in water and creates a pH close to 1, which indicates the strength of its acidity. The molecular structure of nitric acid is as follows:
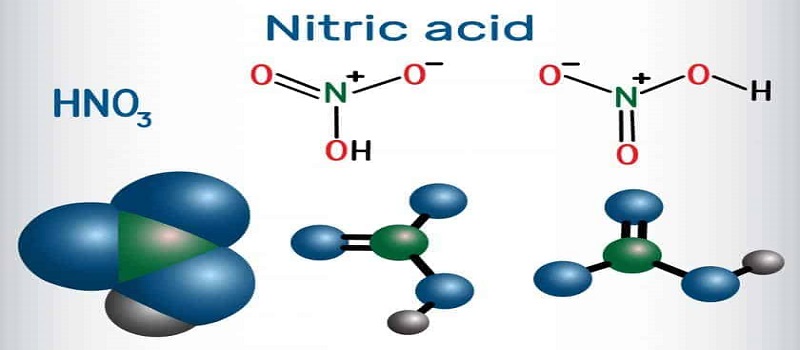
| chemical formula | H 1 N 1 O 3 | Appearance (room temperature) | Colorless liquid |
| Molar mass | 63.01 g/mol | Dissolvability | Completely miscible |
| density | 1.5129 g/cm3 | Refractive index | 1.397 (16.5 °C) |
| melting point | -42 °C | Bipolar torque | 2.17 ± 0.02 D |
| Boiling point | 83 °C | Cas No | 7697-37-2 |
Today, nitric acid is obtained through the Ostwald process, from the oxidation of ammonia in air and with the presence of a platinum catalyst at a temperature of 900 degrees Celsius.
NH 3 (g) + 5O 2 (g) → 4 NO(g) + 6 H 2 O (g)
The nitric oxide obtained from the previous reaction is oxidized again and forms nitrogen dioxide.
NO (g) + O 2 (g) → 2NO 2 (g)
In the next step, nitrogen dioxide combines with water and leads to the production of nitric acid.
NO 2 (g) + H 2 O (l) → 2 HNO 3 (aq) + NO (g)
Another method of producing nitric acid is to use sulfuric acid gas instead of air. To produce nitric acid with this method, furnaces with a temperature of 150 to 170 degrees Celsius are needed. Unlike the previous method, it is more expensive and requires less energy.
Nitric acid can also be prepared through the Brickland method. In this method, oxygen and nitrogen gas are used. But due to the low concentration of nitrogen oxide and the high amount of electrical energy required, it is expensive and there is a possibility of reversing the reaction at high temperature.
Nitric acid has many uses, which we will discuss briefly. If you want to get more comprehensive information about the use of this material, refer to the properties and uses of nitric acid section.
Application of nitric acid in agricultural industry
At present, the most common use of nitric acid is the production of agricultural chemical fertilizers. Due to having nitrogen in its composition, nitric acid has a special advantage for producing fertilizer, and also because of its acidic properties, it causes disinfection and repels pests.
Another application of nitric acid in the agricultural industry is the production of ammonium nitrate. Ammonium nitrate is also one of the important substances in the production of chemical fertilizers.
Application of nitric acid in solvent production
As mentioned, nitric acid has a high acidity property, so it is able to dissolve many substances in itself. For this reason, nitric acid is used in the production of many industrial solvents.
It can also be used to neutralize alkaline environments.
Nitric acid is the only solvent that can dissolve copper and silver, and on the other hand, this acid cannot dissolve gold and platinum. For this reason, in electronic devices where gold is used, copper and silver are separated with nitric acid and the gold remains.
Its other uses are summarized below:
As mentioned, nitric acid is a very strong and industrial acid whose use can be clearly seen in all industries. Therefore, we must learn and practice the safety tips of working with it. Here are the safety tips for working with nitric acid:
Fire: Nitric acid has low flammability, but it catches fire if heat and sparks are created. So it should be kept in a cool place and away from flammable substances.
High acidity: As mentioned, nitric acid has a pH close to 1 and its acidity is so high that touching it will cause irreparable risks. This substance must be stored in a special container, which, in addition to its material, must also have a tight lid so that it does not overflow or gas can escape.
Eye contact: Nitric acid contact with the eyes leads to blindness unless a small percentage of it is in our eyes. If it comes into contact with the eyes, wash the eyes with warm water for 20 to 30 minutes and then consult a doctor.
Contact with the skin: contact of a small amount of nitric acid with the skin of the hand will cause yellow and brown spots and even cause deep and painful wounds on the hand that will remain. If this acid is spilled on the skin (in a small and moderate amount), you should rinse the affected area with hot water for 20-30 minutes. If it comes in contact with the skin of the hand, never splash water on it because it will cause death.
Eating: Eating nitric acid causes heartache, nausea, unconsciousness and even death. If a person is in danger, do not give him anything but water. The amount of water should also be low and around 300 ml, and then consult a doctor immediately.
Inhalation: causes dizziness and breathing and even heart problems. A person who gets poisoned by inhaling nitric acid gas should immediately go outside and use an oxygen capsule. Be aware that the poisoned person's heart rate should be checked regularly and if the heart rate decreases, he should go to the emergency room immediately.
Maintenance: Special containers such as stainless steel or aluminum and glass should be kept, and the surrounding area should be cemented. There should be no metal or alkaline substances near it, and the container should be labeled CORROSIVE-OXIDEZER-POISON.
When working with nitric acid, it is mandatory to use a filter mask, special glasses, long gloves and a long coat. Failure to comply with any of the above will cause irreparable problems.
In this section, we will address the most frequently asked questions about nitric acid:
What are the other names of nitric acid?
Other names are nitric acid, hydrogen nitrate or hydrogen nitrate, azotic acid, brine essence, azotic acid, acid, nitrous acid and anil nitroxide.
What is the decomposition of nitric acid?
Nitric acid at room temperature (25°C) decomposes very little and can be ignored, but if heated and exposed to direct sunlight, its decomposition rate increases and its color changes to red or yellow. It is colored.
Nitric acid reaction with gold and platinum
Because of its high acidity, nitric acid reacts with all metals, but there are only two metals that do not react with nitric acid, which are gold and platinum, which are among the strongest and noblest metals. Therefore, in the extraction of gold from electronic circuits, nitric acid is used to dissolve other metals around the gold wires, and finally the gold wires remain solid in the solution.
How to distinguish gold with nitric acid?
In jewelry that uses gold, it is usually not pure gold and is alloyed with copper and silver. As mentioned above, nitric acid reacts with all metals except gold and platinum. So, if our jewelry is made of gold alloy, the more gold in the alloy, the less reaction it has with nitric acid, and the more impurity our alloy has, the faster and clearer the reaction.
